Density measurement basics
At a glance This section gives you a first insight into the basics of density measurement. You will learn that density is a temperature and pressure-dependent substance property which is often specified with the unit kg/m3 or lb/ft3. The density value is required for determining concentration, average molecular weight and content. For finding the density of gases, it must be noted that this density depends on the respective pressure. The density of liquids depends on the temperature.
Contents
- What is density?
- What do you need density information for?
- Which measuring methods are available for determining the density?
- Comparison of density measuring methods
What is density?
Density is a physical property of a substance that is dependent on temperature and pressure. It provides information on how heavy a substance is. If the mass of two substances of the same quantity is compared, the substance with the higher weight has the higher density. The density ρ (rho) is defined as mass m per volume V.
The density depends on pressure and temperature
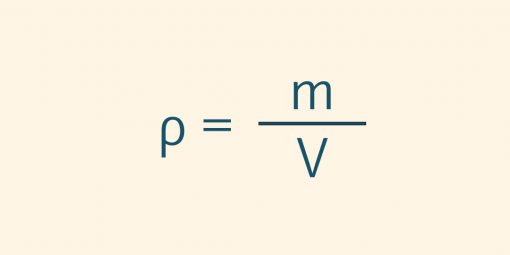
Density formula
Substances can occur as pure substances, mixtures or compounds. If the masses of the individual substances per unit volume are added together, the result is the density of a mixture of substances. The SI unit of density is kilograms per cubic meter (kg/m³). The US unit of density is expressed in pounds per cubic foot (lb/ft³). There are also product or industry-specific units. For example, the degree Oechsle (°Oe) or the degree Brix (°Bx). The units indicate the density or sugar content of must or sugar/water solutions.
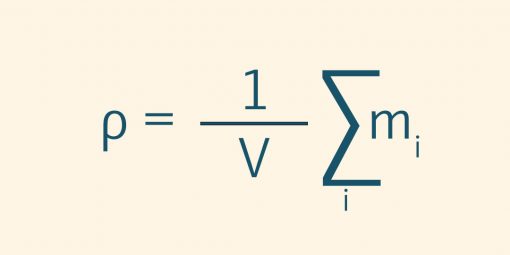
Density formula of mixtures of substances
Conversion of SI units: 1 kg/m³ = 1000 g/m³ = 0,001 g/cm³= 0,000001 kg/cm³
Due to thermal expansion and compressibility, the density of a substance is influenced by the prevailing temperature and pressure. These influencing variables have a greater or smaller effect on the density, depending on whether the substance is a solid or a fluid. The degree of temperature and pressure dependence is much higher for fluids than for solids. In order to obtain a precise density indication, the associated temperature and pressure must be known, especially with fluids.
TrueCalc – Density SI-unit calculator
The volume and the density change with a change in temperature and/or pressure. The mass always remains the same
While the density and volume of a substance change due to the influence of temperature and pressure, the mass always remains constant. If the volume is reduced due to the influence of pressure and/or temperature, while the mass remains constant, the density will increase.
The table shows that the density of gases hardly decreases at a temperature change of one Kelvin. With air, it only decreases by approx. 0.04 kg/m³. With the MEMS chip, such a change might not be clearly detected. However, if the pressure is increased by 1 bar, the gas density in the example of air increases by 1.2 kg/m³. The density of a liquid, on the other hand, hardly changes at all due to a pressure change of 1 bar.
In order to be able to compare substances better, the density of a substance can be converted into a so-called standard density or into a specific density
The standard density (also referred to as “reference density”) indicates the density of a substance or mixture of substances at a certain temperature and pressure. It enables better comparability of different density values with each other. The following standard conditions for temperature tn and pressure pn are frequently used in the listed industries: The standard density of substances or mixtures of substances can be taken from so-called density tables. Examples of density tables can be found here, amongst other sources:
- Alcohol: Standard OIML R 22 “International Alcoholmetric Tables” dated 1973 (http://www.oiml.org/en)
- Sugar: Standard ICUMSA “Densimetry and Tables: Sucrose -Official; Glucose, Fructose and Invert Sugar – Official ICUMSA Method SPS-4” dated 1998 (http://www.icumsa.org)
- Water: PTB notices Wagenbreth, H.; Blanke, W.: “The Density of water in the international system of units and the international practical temperature scale” dated 1968 and Bettin H.; Blanke W.: “The density of water as a function of temperature after the introduction of the 1990 International Temperature Scale
- Gases: NIST DATABASE “NIST Reference Fluid Thermodynamic and Transport Properties Database” (http://www.nist.gov)
The specific density d; also known as relative density, describes the ratio of two density values. The density of a substance is compared with the respective standard density or another reference variable (e.g. air).
The specific density is a dimensionless quantity
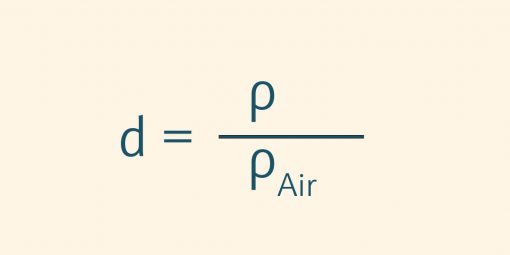
Calculation of specific density d
What are density specifications required for?
Density is a standard value for the characterization of substances and mixtures of substances and is therefore frequently used in the analysis and synthesis of substances. The density value makes it possible to derive various parameters which allow conclusions to be drawn about the composition of a mixture or a compound. Very often the density is used to determine the concentration of a substance in an aqueous solution. The quantitative amount of a (pure) substance in a mixture can be specified in volume percent, mass fraction or as substance quantity concentration. In addition, the quality of a mixture of substances or a compound of substances is often determined by the mean molar mass. The mean molar mass can also be determined with the help of density and enables a characterization of natural gas, for example.
Which measuring methods are available for the determination of density?
There are numerous devices and measuring methods with which the density of a substance can be determined. With the MEMS chip, the resonator density measurement is used as a relatively new principle. Typically, however, even today older measuring methods such as areometer, pycnometer and buoyancy considerations are used.
Measuring methods for the determination of density
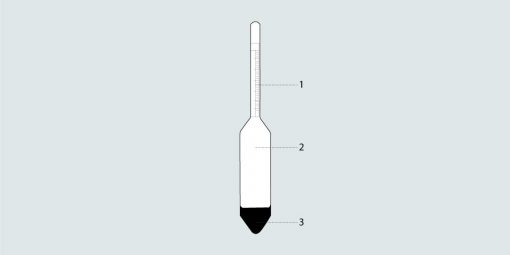
1. Reading scale, 2. Floatation body, 3. Sinker
Areometers are often used for measuring the density of liquids. These glass floats are placed in the liquid and sink until the buoyancy forces of the test liquid are in equilibrium with the areometer weight. The density of the liquid can be derived from the immersion depth of the float.
Source: Liquid density measurement overview articles 2002 Prof. Dr. G. Hradetzky (Merseburg University of Applied Sciences) Prof. Dr. K.-D. Sommer (PTB Braunschweig)

1. Stopper with capillary, 2. Piston with grinding, 3. Sample liquid
Pycnometers are weighing vessels which are first weighed empty and then with the liquid or solid to be measured. The density can be calculated from these two values.
Quelle: http://www.physik.uni-halle.de/Lehre/Grundpraktikum/anleitungen/Pharma-Heft-11.pdf,
Martin-LutherUniversität Halle-Wittenberg
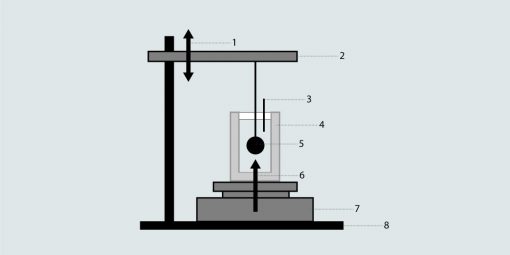
1. Calibration, 2. Lifting device, 3. Temperature mesasurement, 4. Container with liquid, 5. Lifting device, 6. Buoyancy, 7. Balance, 8. Base
With the help of the buoyancy principle, the measurement of density is also possible with buoyancy weighing. A sinking body is immersed in the liquid to be measured and its buoyancy is measured using a balance. The quotient of buoyancy and sinking body volume gives the density of the liquid to be calculated.
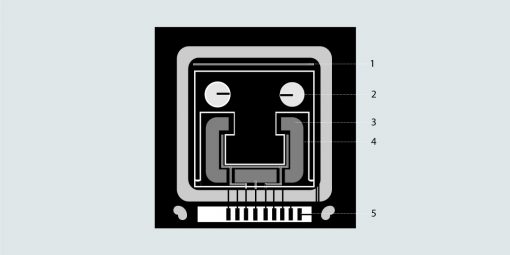
1. Thin film temperature sensorTemperatursensor, 2. Chimney, 3. Measuring channel, 4. Vacuum, 5. Electrodes
The Omega chip is a resonator densimeter. In this method, a resonator moves while maintaining contact with the respective liquid. The vibration frequency is measured, which depends on the density of the liquid.
Comparison of density measuring methods